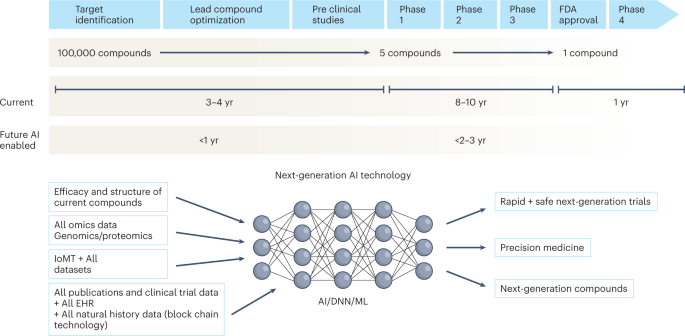
Getting Innovative Therapies Faster to Patients at the Right Dose: Impact of Quantitative Pharmacology Towards First Registratio
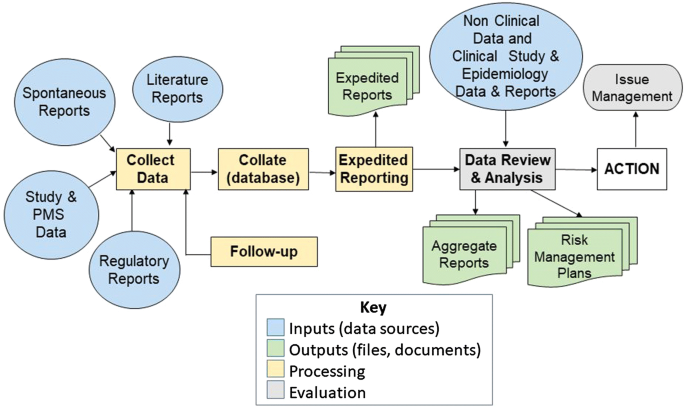
Utilizing Advanced Technologies to Augment Pharmacovigilance Systems: Challenges and Opportunities | Therapeutic Innovation & Regulatory Science
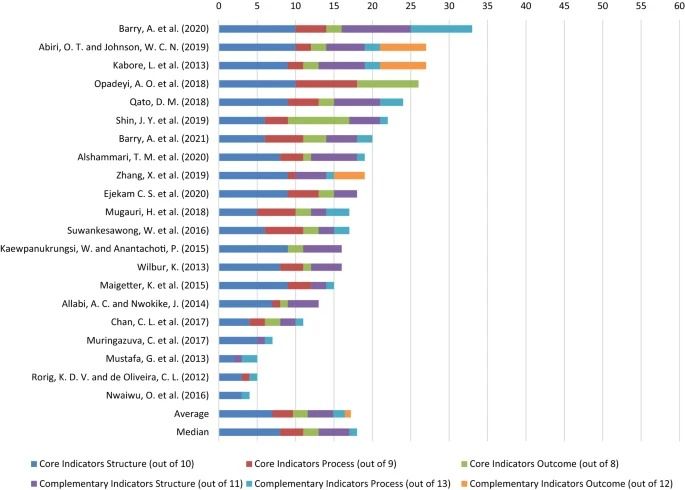
Sistemas de farmacovigilancia en los países en desarrollo Revisión sistemática utilizando los indicadores de farmacovigilancia de la OMS | Fundación Femeba
IJERPH | Free Full-Text | Digital Transformation in Healthcare: Technology Acceptance and Its Applications
TECHNIQUES FOR PREVENTION AND DETECTION OF FRAUD IN RANDOMISED CONTROLLED TRIALS BASED ON ROUTINELY COLLECTED ELECTRONIC HEALT

Applied Sciences | Free Full-Text | Development of a Mobile Application for Smart Clinical Trial Subject Data Collection and Management
Reducing Uninformative IND Safety Reports: A List of Serious Adverse Events anticipated to Occur in Patients with Lung Cancer

A systematic review of models of patient engagement in the development and life cycle management of medicines - ScienceDirect

Bringing Patient and Caregivers Voices to the Clinical Trial Chorus: A Report From the BMT CTN Patient and Caregiver Advocacy Task Force - Transplantation and Cellular Therapy, Official Publication of the American
Assessment of Device Failures and Medication Errors With the Pegfilgrastim On-body Injector in the US and EU
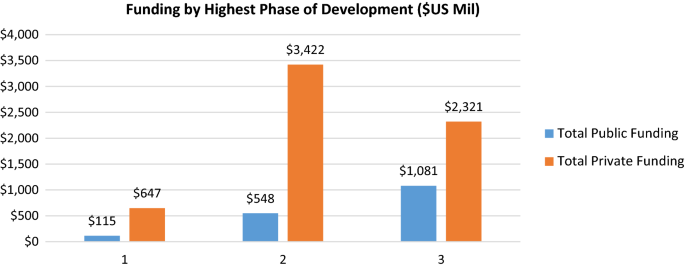
Correction: The Relative Contributions of NIH and Private Sector Funding to the Approval of New Biopharmaceuticals | SpringerLink

Regulatory Science – An Underappreciated Component of Translational Research: Trends in Pharmacological Sciences