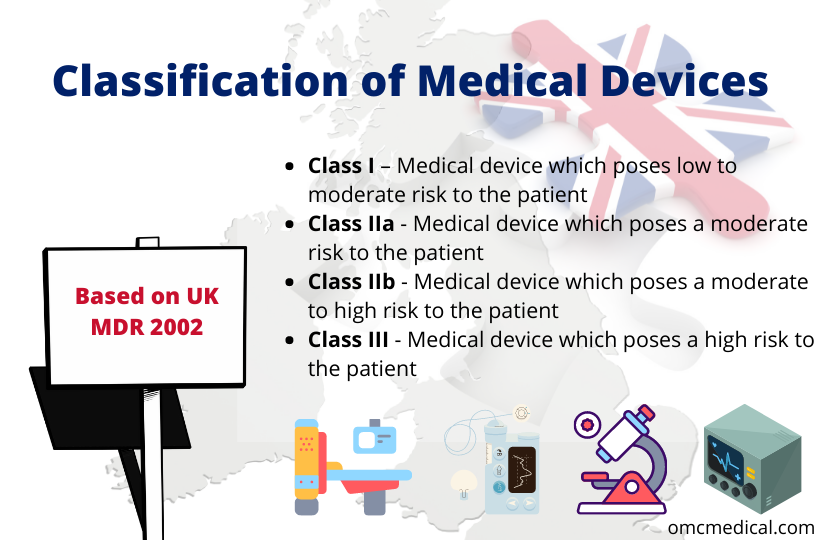
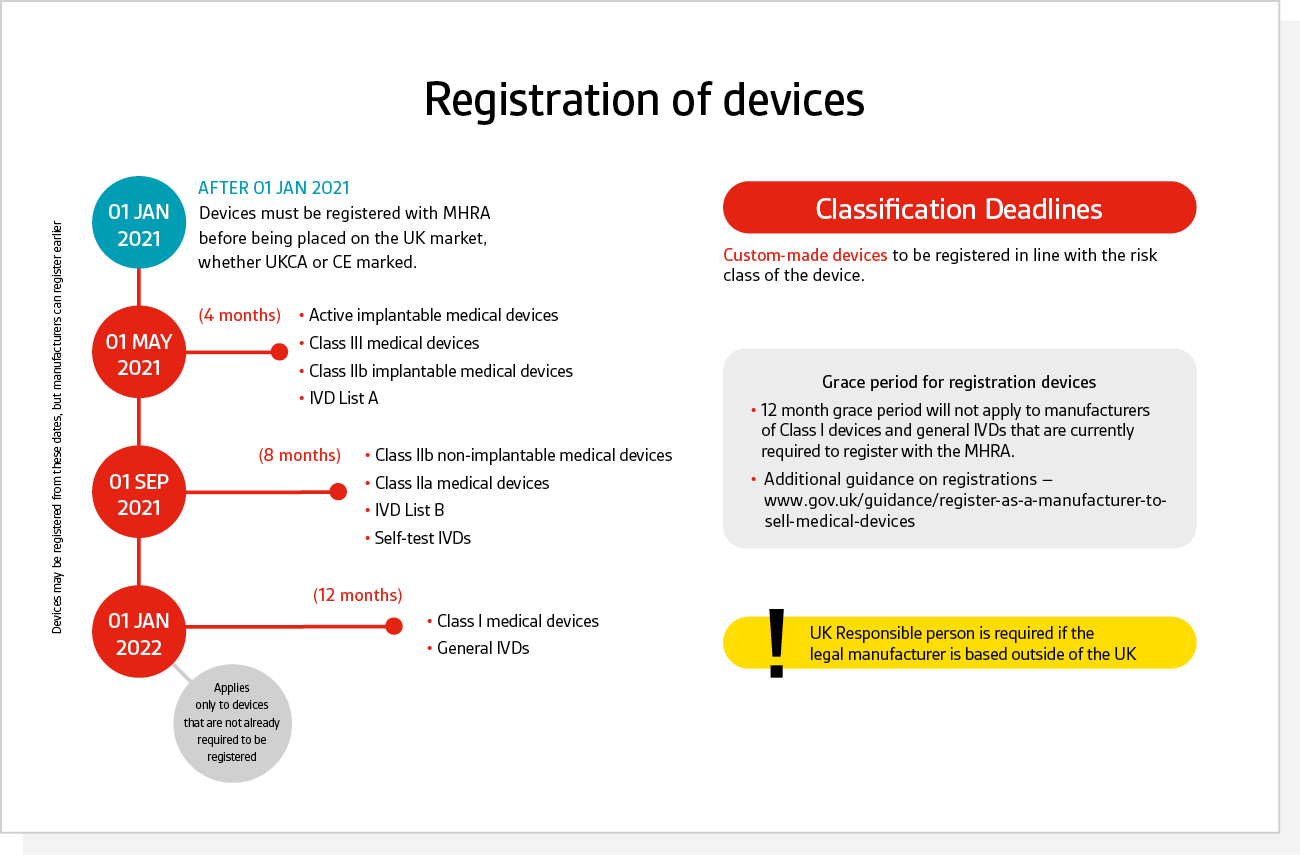
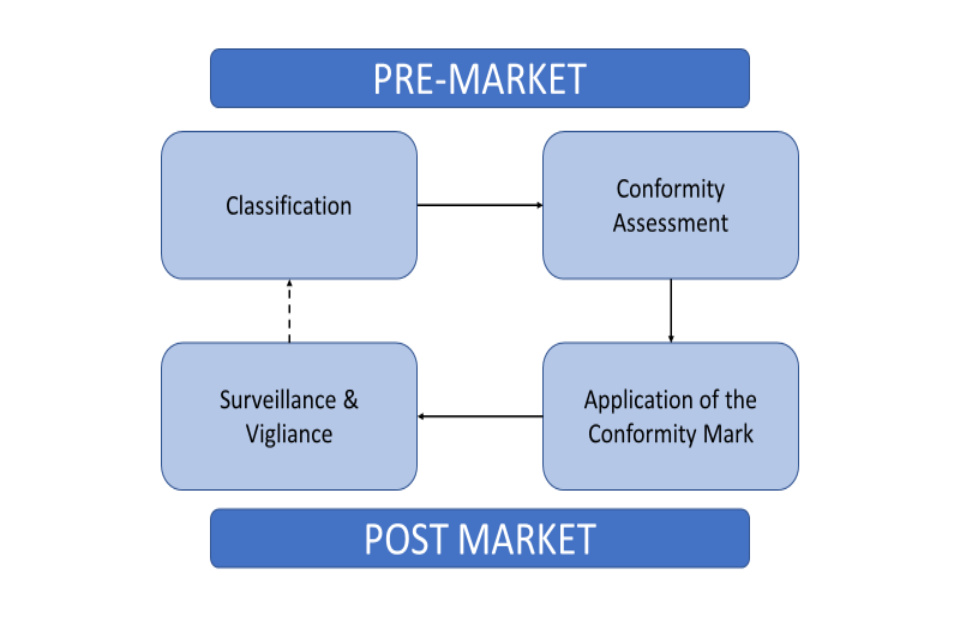
Medical Device “Certificates of Compliance” / “Attestation of Conformity” have no legal standing under UK Medical Device Regulations 2002 - GOV.UK

TÜV SÜD have achieved designation as an Approved Body for the UK Medical Devices Regulations 2002 - YouTube
Dear Dr Wang IN VITRO DIAGNOSTIC MEDICAL DEVICES REGULATIONS 2002: REGULATION 44 Registration of manufacturers of In-Vitro Diagn


%20(1).png)