
Agile Software Development in Regulated Environments Example: Medical Devices | Scaling Software Agility

2022 Executive Certificate Workshop: Regulation of Software as a Medical Device (Conducted Face-to-Face)
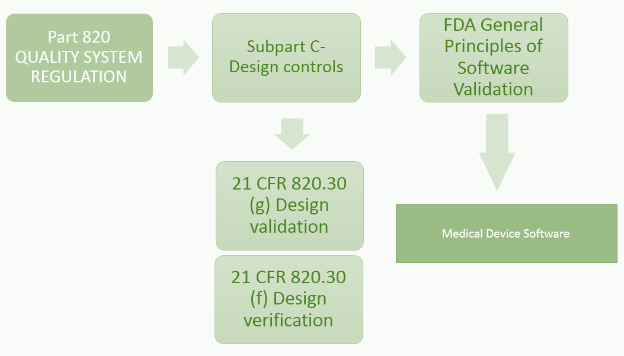
Addressing the Medical Device Software Challenges by understanding FDA's Software Regulation Strategy

Classification of Software as a Medical device under Medical Device Regulation (European Union) - Kvalito

Overview of Regulatory Requirements of Software as Medical Device (SaMD) - Global Regulatory Partners, Inc.

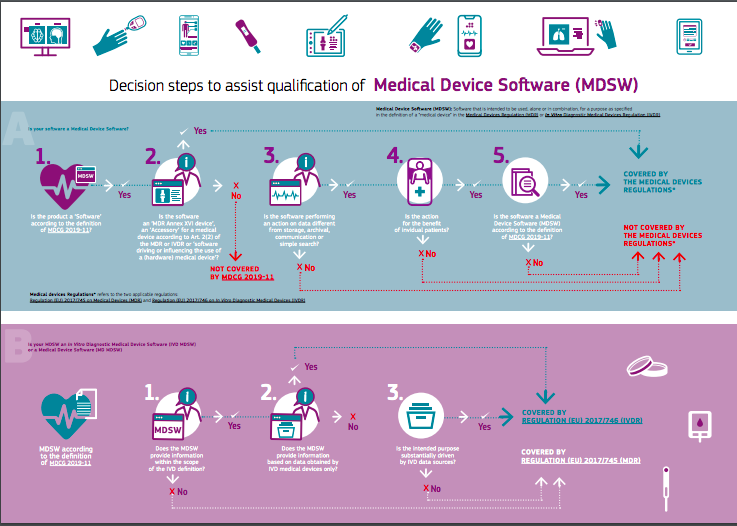
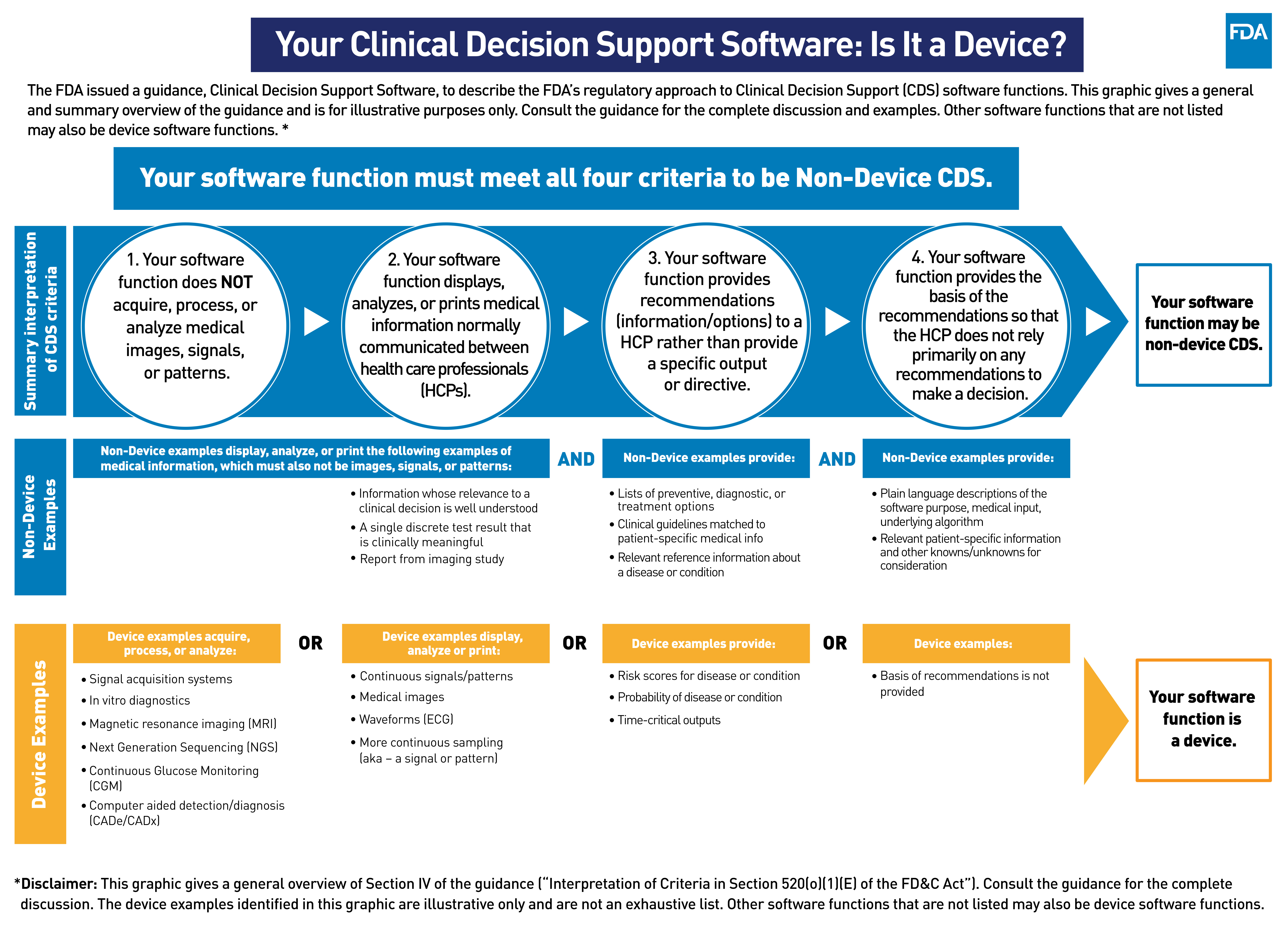
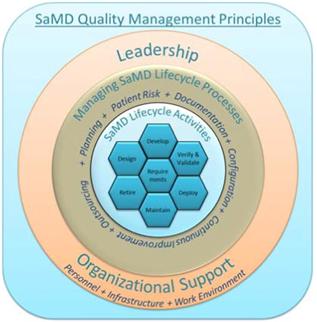
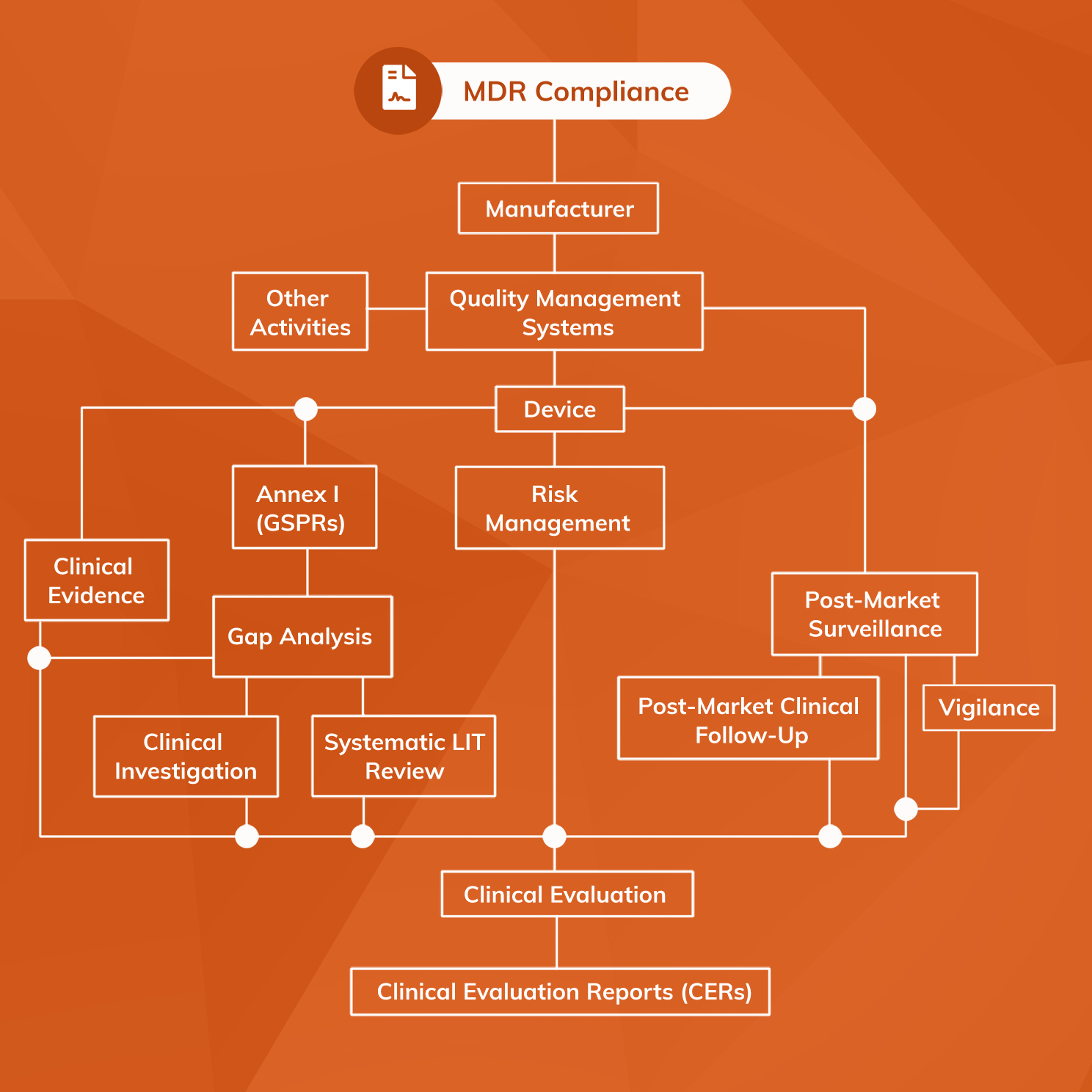
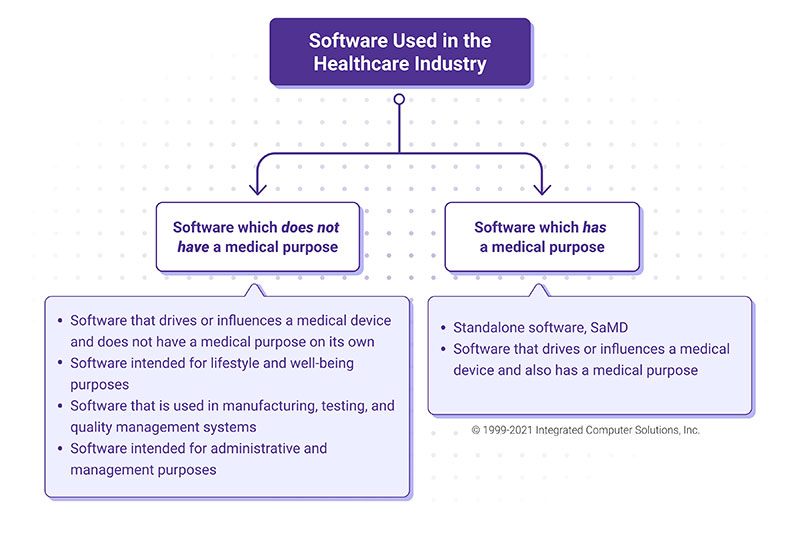



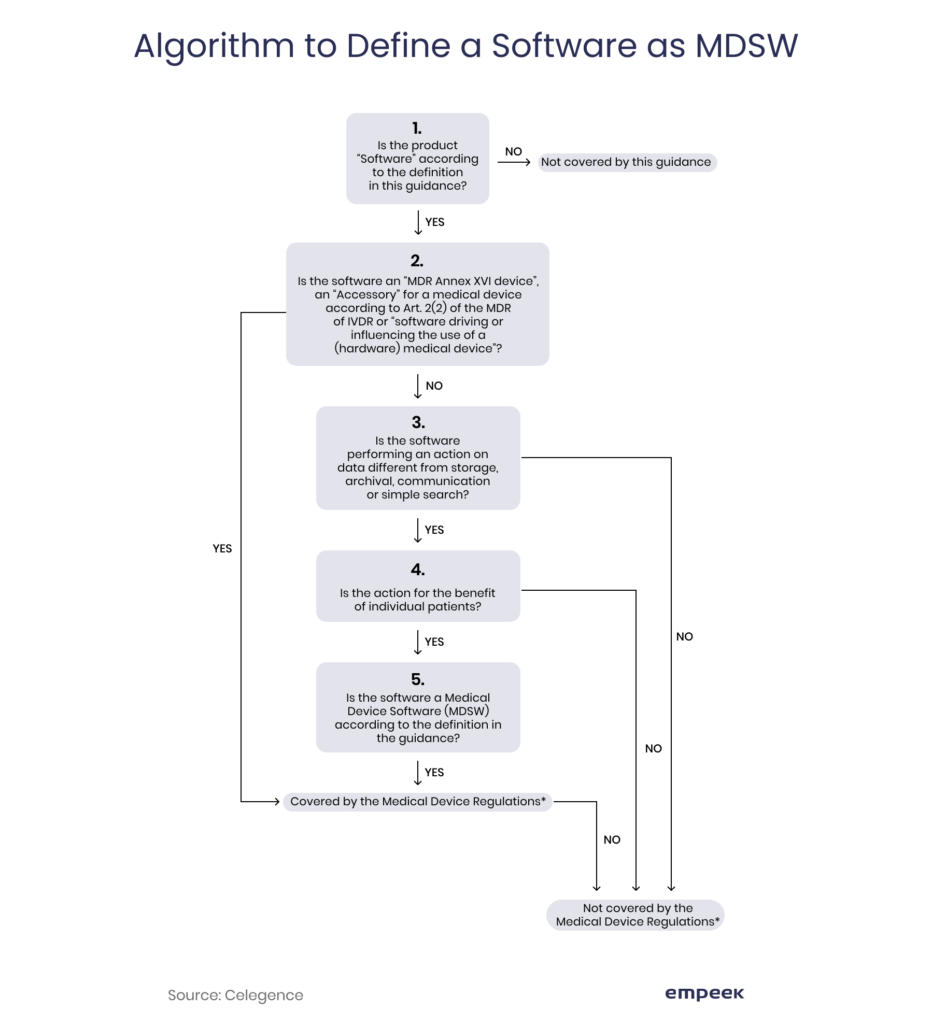

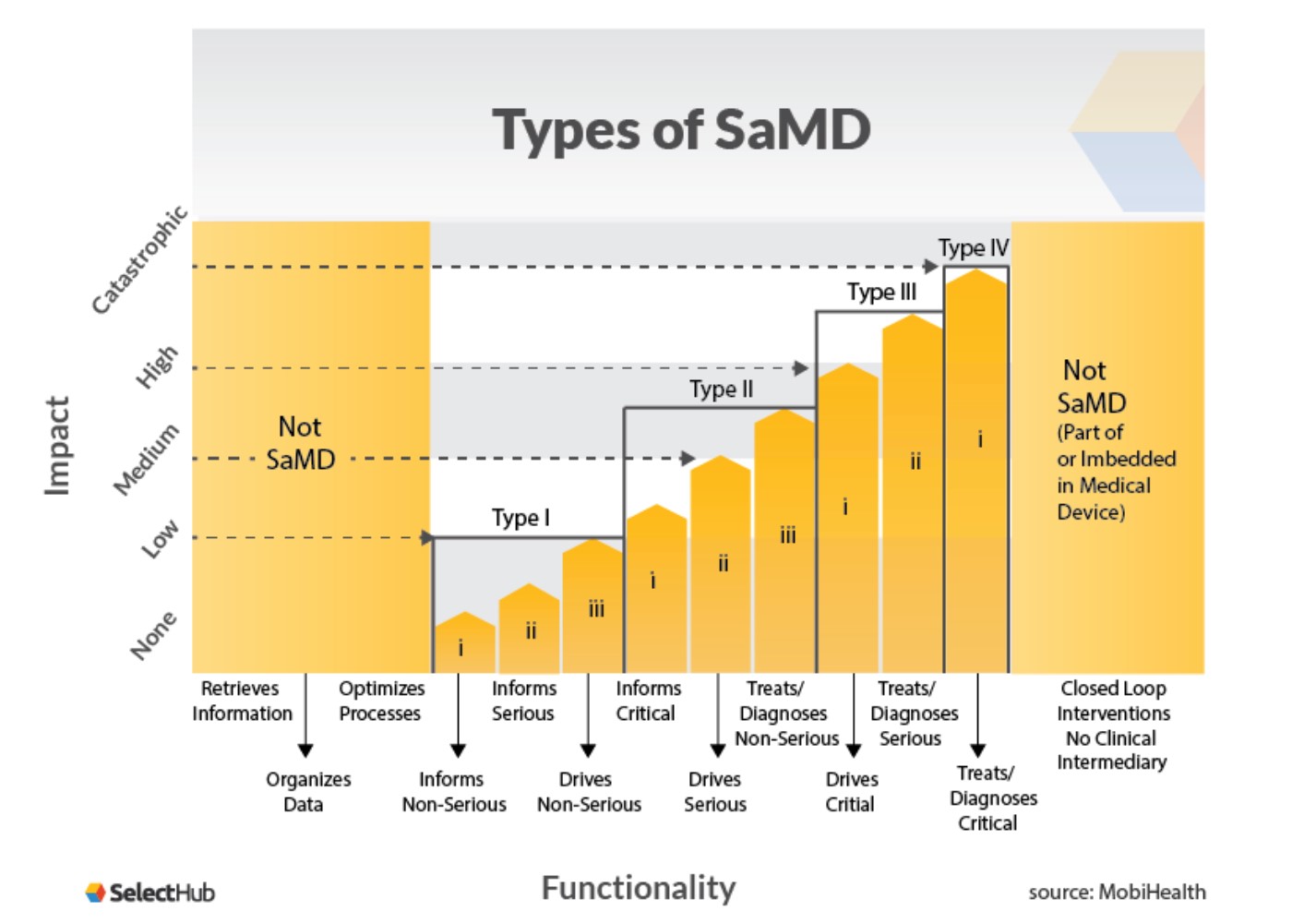
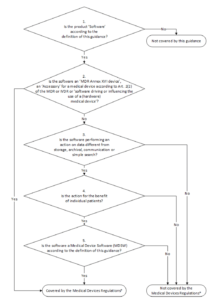
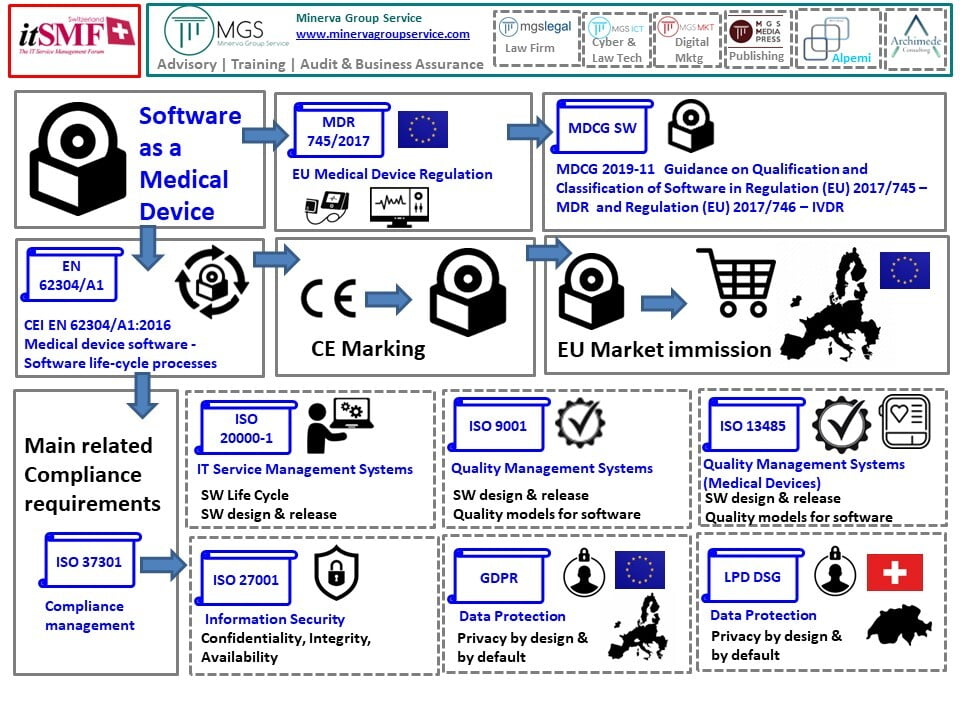
.png?width=800&name=Ultimate%20Guide%20to%20Software%20as%20a%20Medical%20Device%20(SaMD).png)