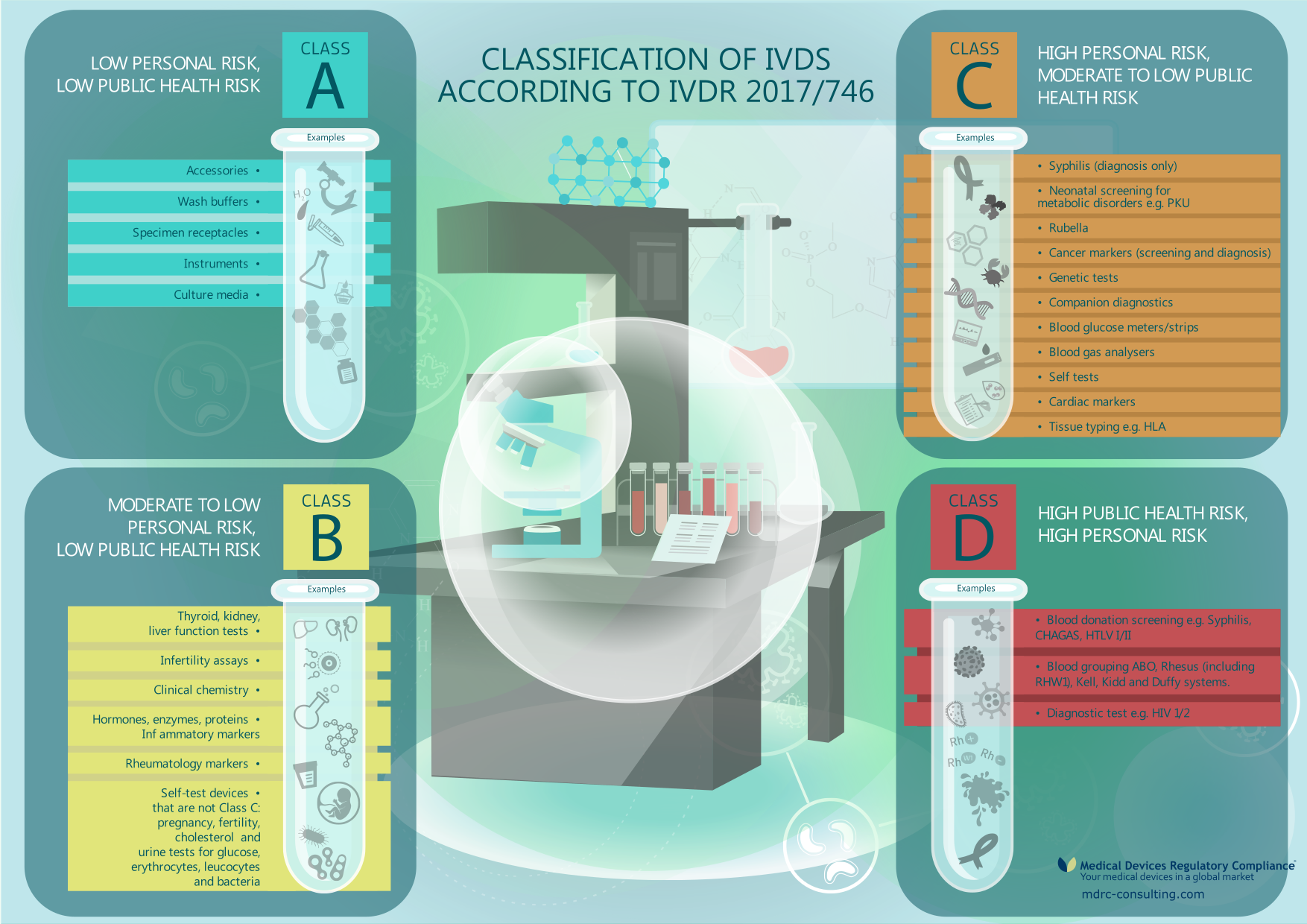
Publicado Manual sobre frontera y clasificación de productos sanitarios bajo MDR e IVDR ver.1 (sept 2022)

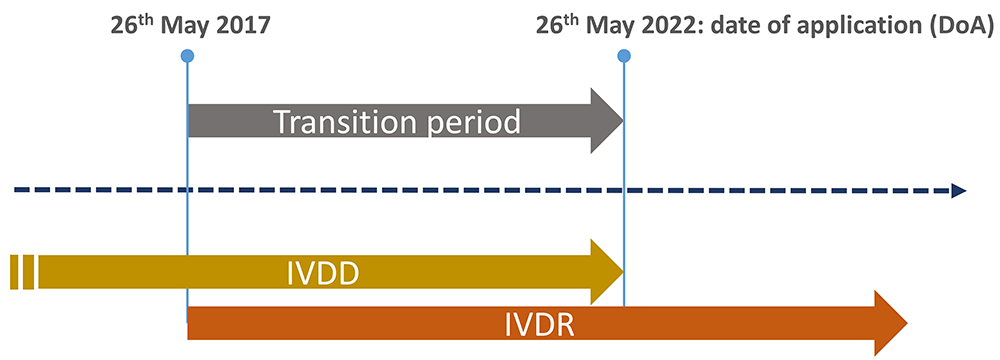
The In vitro diagnostic medical devices regulation (EU) 2017/746: what will change for public health
Key Changes in the Regulatory Requirements for In Vitro Diagnostic Devices Marketed in the European Union Under IVDR 2017/746 - Criterion Edge
Preparation is key: brief checklist how to bring IVD MD into compliance with EU IVDR - Biotech Spain

MDCG 2020-16 rev.2 Guidance on Classification Rules for in vitro Diagnostic Medical Devices under Regulation (EU) 2017/746 - Formiventos

In vitro diagnostic software: Novelties introduced by Regulation (EU) 2017/ 746 - GMED Medical Device Certification
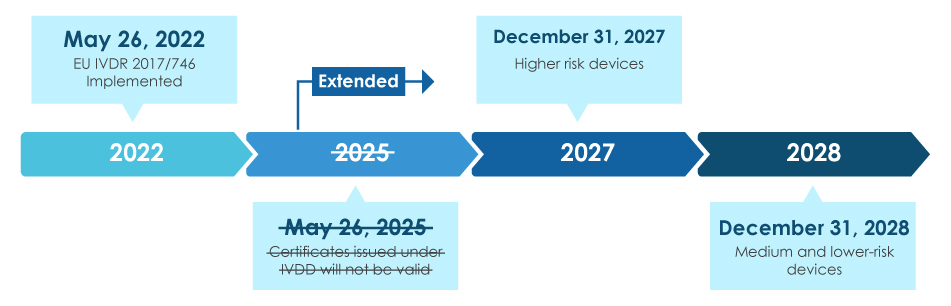
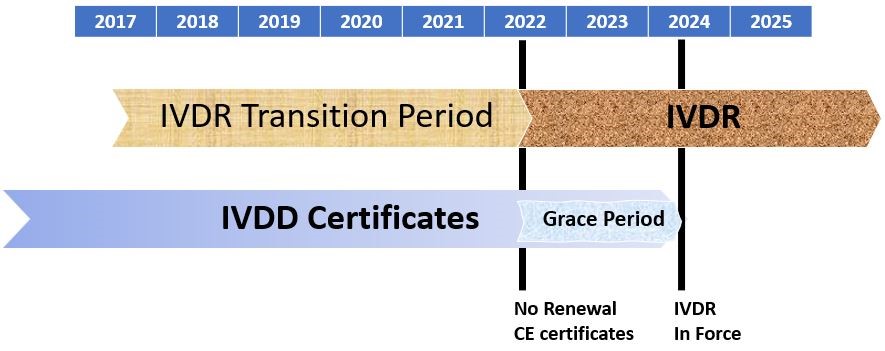
Transition to EU IVD Regulation (EU) 2017/746 and considerations for non-EU regulatory authorities on managing the impact to pro

BfArM - Clinical investigations and performance studies - Performance studies according to IVDR / MPDG